Carbon dioxide (CO2)
Physical and chemical properties
- Chemical formula: CO2
- Relative molecular mass: 44
- Gas constant: 189 J/kg·K
- Boiling/sublimation point at 101.3 kPa: -78.4°C (this temperature corresponds to change in phase between solid and gaseous state)
- Critical temperature: 31.1°C
- Critical pressure (abs.): 73.6 bar
- Solubility in water: up to 88 ml in 100 g H20 at 20°C
Impact on human beings and environment
Carbon dioxide is A1 safety group: nontoxic (low-toxic), inflammable. In high concentration, CO2 is an asphyxiating gas. Carbon dioxide is heavier than air, so when leaked in closed premises it accumulates at the floor level.
According to GOST EN 378-1-2014, the oxygen threshold value (oxygen deficit limit) is 0.07 kg/m3, the practical limit for occupied spaces is 0.1 kg/m3.
The practical limit refers to the concentration of a refrigerant that does not harm a human being or require immediate evacuation in case of casual leakage and release of all the volume into the room. The practical limit is used to specify the maximum refrigerant charge of a specific refrigeration plant.
Another risk consideration for CO2 is high working pressure, so to prevent accidents, safety valves and pressure switches are used.
Carbon dioxide’s ODP is zero, and its impact on climate is taken as a unit of global-warming potential (GWP). GWP of many traditional refrigerants—CFC, HCFC, HFC—is hundred and thousand times higher than that of CO2.
Production of carbon dioxide
In industry, carbon dioxide is generated from stack gases or as a by-product of various chemical processes, e.g. decomposition of natural carbonates or production of alcoholic drinks.
CO2 can be a by-product of generating pure oxygen, nitrogen, and argon at air separation units.
Use of carbon dioxide
The refrigeration industry has been using carbon dioxide since the XIX century. According to the international nomenclature (R-numbering), CO2 is referred to as R-744.
Carbon dioxide has high thermal conductivity, relatively low viscosity, low critical point, and high triple point. The gas density ensures high heat transfer with air. The pressure loss in the pipeline almost does not influence the efficiency of cooling due to high working pressure. Due to the high volumetric capacity, CO2 systems are more compact.
Transcritical and subcritical refrigeration cycles
At atmospheric pressure, carbon dioxide exists only in a solid or gaseous phase: it is called dry ice at temperatures below -78.4°C, and at higher temperatures evaporates.
At the triple point—at 5.2 bar and -56.6°C—carbon dioxide has three phases at equilibrium.
The critical point is at 31.1°C at 73.8 bar, and with temperature rise, carbon dioxide turns into a gas (supercriticality).
CO2 refrigeration cycle with a range of working temperatures and pressures below the critical point and above the triple point is a subcritical cycle. It is a standard refrigeration cycle of a vapor compression refrigerating machine where heat is transferred from a cooler body (medium) to a warmer one through the change in the refrigerant aggregate state accompanied by heat absorption or release.
CO2 refrigeration cycle with heat abstraction at temperatures above the critical one (31.1°C) and without condensing is a transcritical cycle. Since no condensation takes place, the heat exchanger where the refrigerant releases heat in the environment is not a condenser but a gas cooler.
Transcritical refrigerating plants
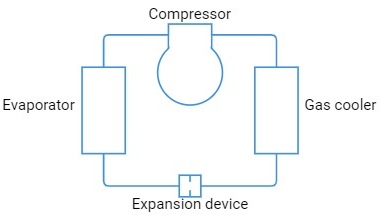 Basic transcritical refrigerating plant
Basic transcritical refrigerating plant
The basic transcritical plant consists of a compressor, gas cooler, evaporator, and expansion device.
The basic plants are not fitted with pressure controls; they operate at optimum high pressure and maximum output under constant conditions.
In more complex systems, a thermal valve regulates the cooling temperature, and a low-pressure receiver compensates the load swing at the high-pressure side. Heat exchange in such systems is arranged between the compressor suction line and the gas cooler discharge line.
Recently, many different CO2 transcritical refrigeration units have been developed, including compact packaged screw compressors for fishing vessels.
Transcritical cycle can also be used in air-water heat pumps developed in Japan and consuming 68% less electrical energy than electrical heaters to heat water to 90°C. Air-water CO2 heat pumps are environmentally friendly because they use low-GWP refrigerants, consume less energy, and heat water without burning fossil fuels.
Transcritical booster (two-stage) systems
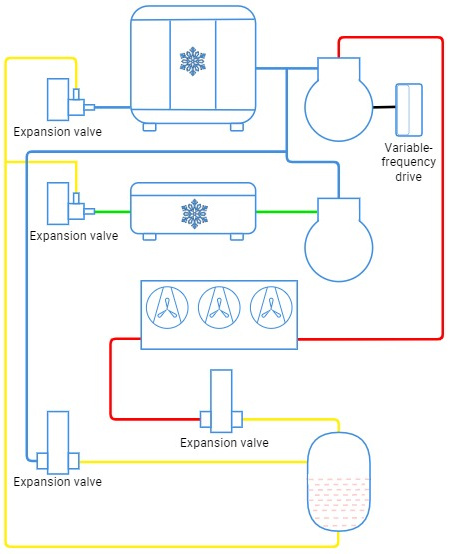 Transcritical booster system for commercial use
Transcritical booster system for commercial use
Low-temperature systems used for, e.g. freezing, operate at the high discharge temperature that is reached by a two-stage (booster) compression where the refrigerant (CO2) is discharged by the low-temperature compressor to the suction port of the medium-temperature compressor. To cool the gas between two compressors, throttling expansion of the liquid refrigerant from the high-temperature stage, and the gas released from the receiver through the pressure-control valve is applied.
Cascade refrigerating plants and secondary refrigerant systems
As separate plants, CO2 refrigerating plants operating only in the subcritical cycle are not widespread; they are used as low-temperature stages of cascade systems. The heat released during condensation of carbon dioxide is absorbed by the evaporating refrigerant of the high-temperature circuit (usually ammonia). Examples of NH3/CO2 systems are described in a separate section.
There are systems with CO2 used at two stages: the high-temperature stage operating in the subcritical cycle, and the low-temperature stage, can operate in the transcritical cycle if the ambient temperature is high.
Carbon dioxide can serve as a secondary refrigerant. It is cooled by a basic refrigeration machine using synthetic or natural (hydrocarbons, ammonia) refrigerants, and is pumped by a centrifugal pump. An example of a system with the ammonia chiller and carbon dioxide as a secondary refrigerant is described in a separate section.
As a secondary refrigerant, carbon dioxide has the advantage of requiring a pump of smaller capacity, and high volatility, so absorption of latent heat in the consumer’s heat exchanger results in partial evaporation of CO2 already there.
Additional materials
- Stefan Jensen of Scantec Refrigeration Technologies details the roadblocks facing natural refrigerants in Australia
- CARB ‘regulatory concept’ would set a refrigerant GWP limit of 5 for transport refrigeration units
- Jordan’s National Cooling Strategy сalls for natural refrigerants transition in RAC sector
- Enex integrated CO2 rack and heat pump cuts Polish hypermarket’s annual emissions by 70 tons
- EU F-gas law leads to natural refrigerant solutions for non-Traditional HVAC&R industries
- Japanese frozen tuna warehouse with new air and R717/CO2 refrigeration systems cuts summer energy use by 15% over R22 and R23
- French ice rink expects up to 35% energy savings with CO2 compared to R404A
- Güntner finds transcritical CO2 to be up to 18.6% more efficient than R404A in Monterrey, Mexico
- KIO Networks says its new Valencia data center is Spain’s first to use 100% natural refrigerants
- Study finds R717/CO2 cascade refrigeration system offers 35% higher COP than R404A systems to Indian seafood processors
- Cool Talks: “We need to inform, through different channels, that there are alternatives to F-gases and that these alternatives are not dangerous”
- Enex launches ‘first-ever’ transcritical CO2 flat gas cooler rated to 140bar
- Carrier/GIZ Collaboration to Provide CO2 Reefer Training in Costa Rica and South Africa
- ATMOsphere releases 2023 market report showing robust growth of transcritical CO2
- Market demand for natural refrigerant training is rising, says Austrian Association
- Experts call for more natural refrigerant case studies
- Hillphoenix director counters efficiency and operational criticisms of CO2 refrigeration
- Automated cold-storage facility in Canada to employ integrated CO2 system
- Combined cooling, heating and power systems can boost supermarket eficiency, says contractor Neelands
- CO2 defrost system cuts energy use by 20% for Japanese distributor Kyodo Suisan Ryutsu
- Copeland, Hillphoenix and AAA Refrigeration demonstrate the power of collaboration in advancing CO2 adoption
- Energy Recovery, ALDI US, Hydro-Québec and Pega Hrnjak win ATMO Awards America 2023
- Danish utility poised to supply 60MW of zero-emissions district heating with MAN’s CO2 heat pumps
- Study finds that cascade heat pump using mixtures of R744/R600 and R744/R601 produce COP of 4.5 and hot water above 100°C
- Study finds ‘novel’ transcritical CO2 ejector system outperforms conventional CO2 system up to 82.5% in hot climate
- CO2 refrigeration helps cut electricity use at finnish ice rink by 34%
- Danfoss introduces a new algorithm for enhanced control of ejectors and high-pressure valves in a CO2 industrial system
- Scientist calls for use of natural refrigerants in ice slurry systems on fishing vessels
- Demonstration sites highlight potential of natural refrigerants in India
- Energy Recovery’s pressure exchanger boosts Epta CO2 refrigeration efficiency by over 30% at ambient temperatures above 40°C
- ‘Hybrid ejector’ found to boost efficiency of transcritical CO2 refrigeration by up to 42%
- Advansor launches CO2 rack for smaller urban supermarkets
- Packaged ammonia or CO2? Evapco now offers both
- Start promoting HVAC&R industry to younger students, IIAR panelists urge
- METRO continues rollout of transcritical CO2 in all new stores and refurbishments
- ATMOsphere study finds 1,895 transcritical CO2 sites in North America, 6,960 in Japan
- Transcritical CO2 system’s efficiency boosted with hydrocarbon heat pump in Netherlands
- ATMOsphere estimates 55,000 stores using transcritical CO2 in Europe
- Gas cooler control needed for efficient transcritical CO2
- Global Online Database ‘Cool Technologies’ Seeks Examples of R744-Based Products and Installations
- CTS Research Firm Shows Versatility of CO2
- Evapco Reports Installation of 100 Low-Charge Ammonia Packaged Units at 25 Sites
- Refrigerant Carbon Credits Plan Aims at Cutting Cost of NatRef Transition
- The road to zero impact: the final data of Epta Life-C4R project
- Industry refrigerant guidance outlines system efficiency and safety challenges
- ATMO World Summit: CO2 is ‘Close to the Ideal Refrigerant’
- Bulgarian OEM Schiessl Provides Low-Temp CO2 Racks Linked to R290 Chillers for German Warehouse
- Food-Packing Company Saves 30% in Energy with CO2 Brine Chiller
- Koura submits CO2 alternative for ASHRAE approval
- Results of the conference "Refrigeration Industry 2021": the future belongs to natural refrigerants (in Russian)
- Frascold Upgrades CO2/R290 Compressor Test Laboratory
- French Seafood Processor Saves Up to 36% in Energy With CO2 Cooling/Heating
- Norwegian Researchers Develop Simplified Ejector-Based CO2
- Natural refrigerants in North America. Industry and special uses (in Russian)
- OzonAction webinar: “Refrigerants, naturally!” against HFO (in Russian)
- New CO2 transcritical system in Lithuanian supermarket: energy consumption reduced by 30% (in Russian)
- Two luxe Volkswagen using CO2 (in Russian)
- MAC directive. Daimler insists on using CO2 and rejects the safety of R-1234yf (in Russian)
- Natural refrigerants for the future of Russia (in Russian)
- Refrigerants and environment (in Russian)
- ATMOsphere Asia 2014 opens the road to innovative solutions based on natural refrigerants in Japan, China, and South-East Asia (in Russian)
- Energy and environmental paradigms of refrigerants (in Russian)
- 26.01.2016, Press conference “Russian refrigeration industry and global environmental agreements”
- Daimler continues using CO2 systems and disagrees with JRC’s report on R-1234yf (in Russian)
- Brazil’s booming economy boosts the carbon dioxide refrigeration sector (in Russian)
- HFC taxes and financial incentives for transfer to natural refrigerants in the EU (in Russian)
- Natural refrigerants in North America. Food supply (in Russian)
- Brazilian supermarkets choose CO2 (in Russian)
- Natural refrigerants come to commercial refrigeration (in Russian)
- Pros and cons of using eco-friendly refrigerants in refrigeration plants (in Russian)
- First Russian freon-free grocery using ozone-safe refrigerant CO2 (in Russian)
- Propane and carbon dioxide reduces hop production cost (in Russian)
- IIAR 2014: CO2 triumph in Brazil (in Russian)
- Coca-Cola installs 1 millionth carbon dioxide refrigerator (in Russian)
- Demonstration project of a CO2 refrigerating plant for a retail store (in Russian)
- 24.11.2015, Workshop on organization of production of carbon dioxide refrigeration equipment. Working model of a CO2 refrigerating plant for a retail store
- Europe: focus on training specialists in working with new refrigerants (in Russian)
- Ozone-depleting substances and environmentally safe alternatives (in Russian)
- Natural refrigerants in North America. Industry and special uses (in Russian)
- About natural refrigerants (in Russian)
- Natural refrigerants in North America. Transport (in Russian)
- Carbon dioxide for automobile air conditioners (in Russian)


